
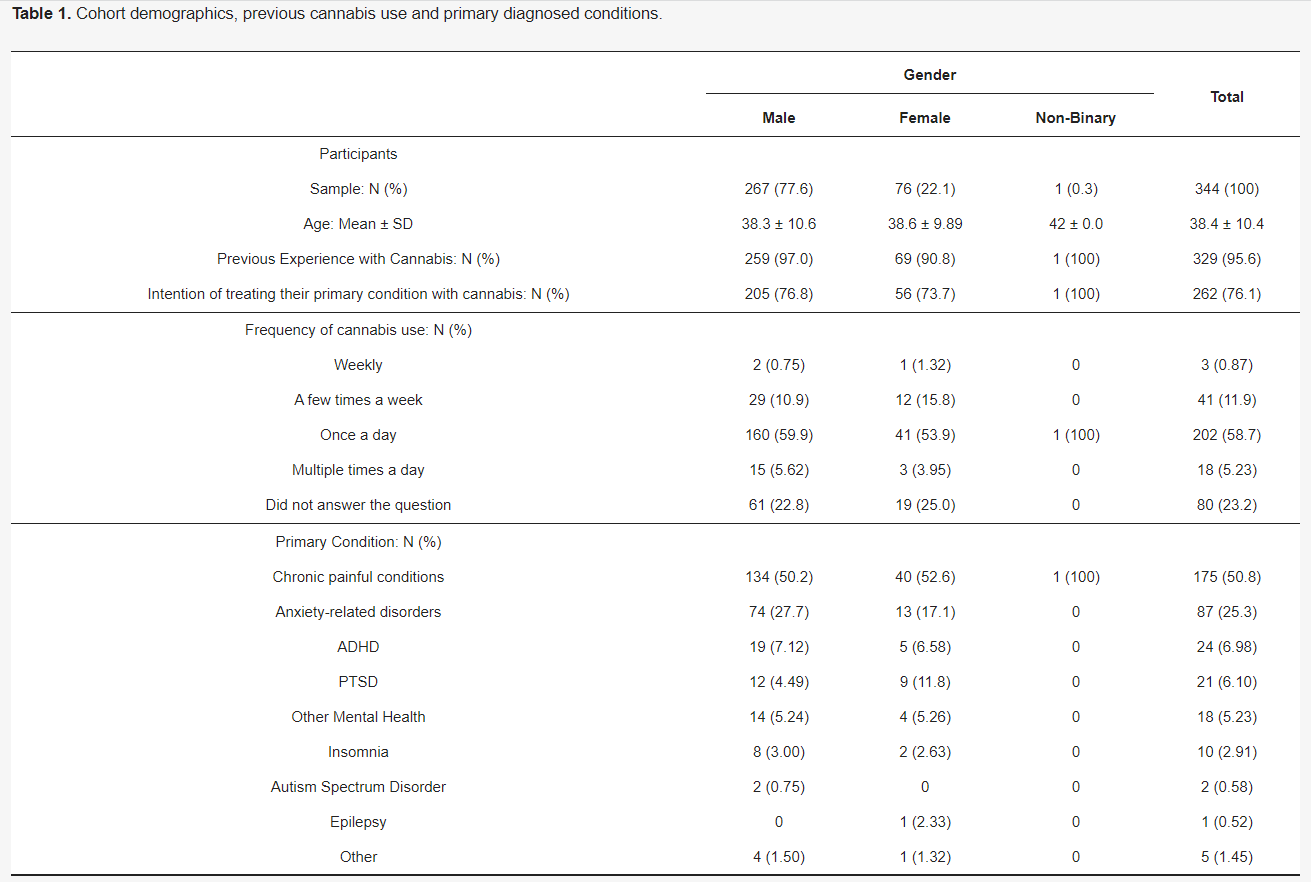
Abstract
In November 2018, the UK’s Home Office established a legal route for eligible patients to be prescribed cannabis-based products for medicinal use in humans (CBPMs) as unlicensed medicines. These include liquid cannabis extracts for oral administration (“oils”) and dried flowers for inhalation (“flos”). Smoking of CBPMs is expressly prohibited. To date, THC-predominant cannabis flowers remain the most prescribed CBPMs in project Twenty21 (T21), the first multi-center, prospective, observational UK cannabis patient registry. This observational, prospective data review analyzes patient-reported outcome measures (PROMS) collected by T21 associated with the inhalation of KHIRON 20/1, the most prescribed CBPM in the project. PROMS collected at baseline and at subsequent 3-month follow-up included health-related quality of life (HRQoL), general mood, and sleep. Condition-specific measures of illness severity were performed with the Brief Pain Inventory Short Form (BPI-SF) and the Generalized Anxiety Disorder 7-Item Scale (GAD-7). Participants (N = 344) were mostly males (77.6%, average age = 38.3) diagnosed mainly with chronic pain (50.9%) and anxiety-related disorders (25.3%). Inhalation of KHIRON 20/1 was associated with a marked increase in self-reported HRQoL, general mood, and sleep (N = 344; p < 0.001). Condition-specific assessments showed significant improvements in pain severity (T = 6.67; p < 0.001) and interference (T = 7.19; p < 0.001) in patients using KHIRON 20/1 for chronic pain (N = 174). Similar results were found for patients diagnosed with anxiety-related disorders (N = 107; T = 12.9; p < 0.001). Our results indicate that controlled inhalation of pharmaceutical grade, THC-predominant cannabis flos is associated with a significant improvement in patient-reported pain scores, mood, anxiety, sleep disturbances and overall HRQoL in a treatment-resistant clinical population.
Results
Results indicate that controlled inhalation of pharmaceutical grade, THC-predominant cannabis flos was associated with a robust improvement in patient-reported pain scores, general mood, anxiety, sleep, and overall HRQoL in a treatment-resistant clinical population. The effect size, which was larger in patients diagnosed with anxiety disorders compared to chronic pain, appeared to be maximal at 3 months and sustained for at least 6 months. Occurrence of side effects was minimal, probably due to the previous experience of participants with cannabis inhalation. This evidence supports the notion that the administration of cannabis flos in a medicalized environment under the supervision of a trained healthcare provider further improves the clinical outcomes of legally prescribed CBMPs when compared to chronic patients self-medicating with illegal cannabis.
Clinical experience with CBD: dried flower and oral extracts
References
-
- O’Shaughnessy, W.B. New remedy for tetanus and other convulsive disorders. Lancet 1840, 34, 539–541. [Google Scholar] [CrossRef]
- House of Lords—Science and Technology—Ninth Report. Available online: https://publications.parliament.uk/pa/ld199798/ldselect/ldsctech/151/15101.htm (accessed on 28 July 2022).
- Rescheduling of Cannabis-Based Products for Medicinal Use in Humans (Accessible Version)—GOV.UK. Available online: https://www.gov.uk/government/publications/circular-0182018-rescheduling-of-cannabis-based-products-for-medicinal-use-in-humans/rescheduling-of-cannabis-based-products-for-medicinal-use-in-humans-accessible-version (accessed on 28 July 2022).
You can find the complete article from the MDPI from 14.10.2022 here: https://www.mdpi.com/2227-9059/10/10/2576